Background
The research report “Global cancer burden growing, amidst mounting need for services” released by the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO), shows that in 2022, there were 20 million new cancer cases and 9.7 million cancer deaths worldwide, with lung cancer being the most common new case and cause of death globally. It is estimated that by 2050, the global incidence of new cancer cases will exceed 35 million, representing a staggering 77% increase compared to 2022. The emergence of multi-cancer early screening has epoch-making significance for the development of cancer screening and diagnosis. Compared to single-cancer detection, multi-cancer detection can achieve simultaneous detection of multiple cancers (including several cancers for which screening methods are not yet recommended), making it an inevitable trend for the future of the industry. With the development of the cancer early screening industry and the expansion of market, an increasing number of companies are now entering the multi-cancer early detection market, transitioning from single-cancer to multi-cancer detection. Therefore, Apostle has launched the Apostle MiniEnrich EMS Panel (v1.0), with integrates the latest upgrades of Apostle MiniEnrich Hybrid Capture Reagents v2 and Apostle MiniEnrich NanoBlockers. Based on the new Apostle MiniEnrich Methylation Hybrid Capture System, which can capture and convert methylated libraries, covering the methylation status targets of candidate genes for target cancer species within the test samples.
Introduction
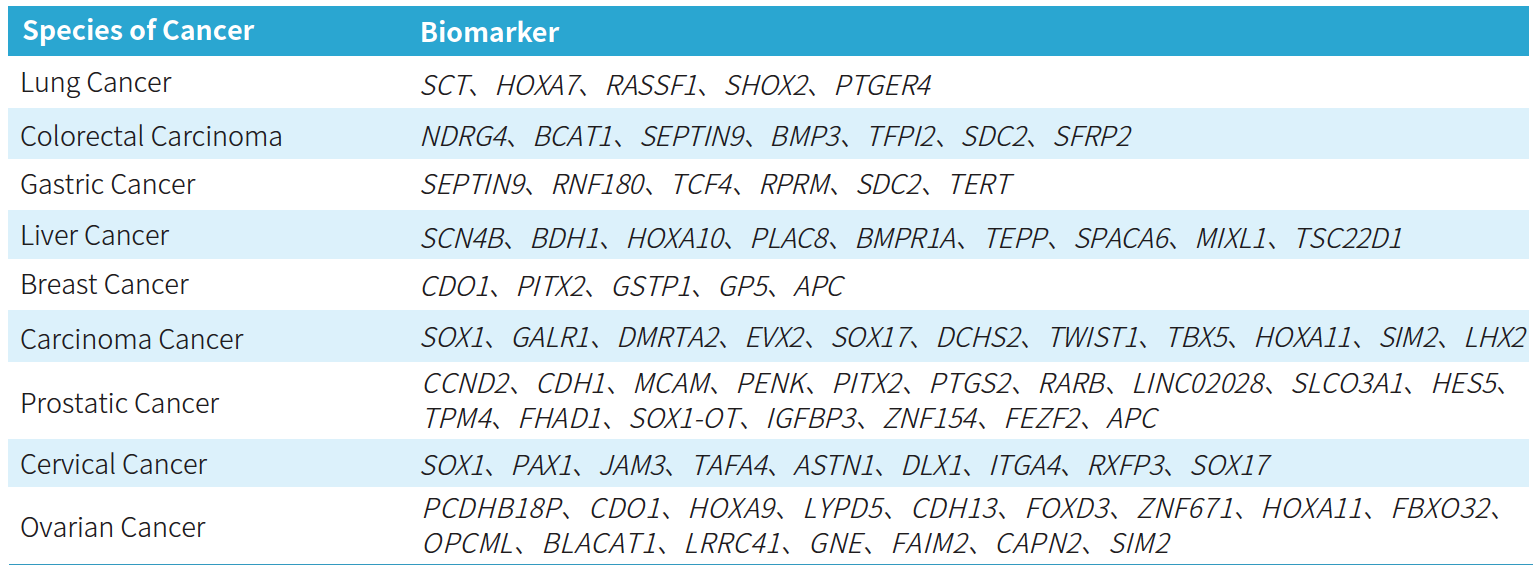
Apostle MiniEnrich EMS Panel v1.0 (Early Methylation for Screening) covers methylation gene sites related to nine major high-incidence cancers, including selected sites approved by the NMPA and FDA, as well as those reported in literature and patents. It encompasses nine types of cancers, including lung cancer, colorectal cancer, gastric cancer, liver cancer, breast cancer, carcinoma cancer, prostate cancer, cervical cancer, and ovarian cancer, involving 76 methylated candidate genes associated with carcinogenesis and tumor suppression, with over 2,000 CpG sites. The probe design covers approximately 20 Kb of the human genome, providing comprehensive and accurate support for methylation early screening.
Product Features
o Multi-Cancer Detection: Covers CpG sites of multiple genes for 9 major cancer types in a single detection.
o Sample Compatibility: Suitable for gDNA, cfDNA, and various level of FFPE samples.
o Accurate Quantification: Achieves precise quantification of methylation levels in tested samples.
o Efficient Capture: Combines with Apostle MiniEnrich Total Solution for Methylation to ensure uniform data quality, higher capture efficiency and stability.
o High Reliability: Utilizes exclusive patented probe design to comprehensively detect methylation status, providing reliable results.
o Flexible Customization: Customizes panels containing different genes and scales of CpG sites for testing specific single/multiple cancer types.
Performance
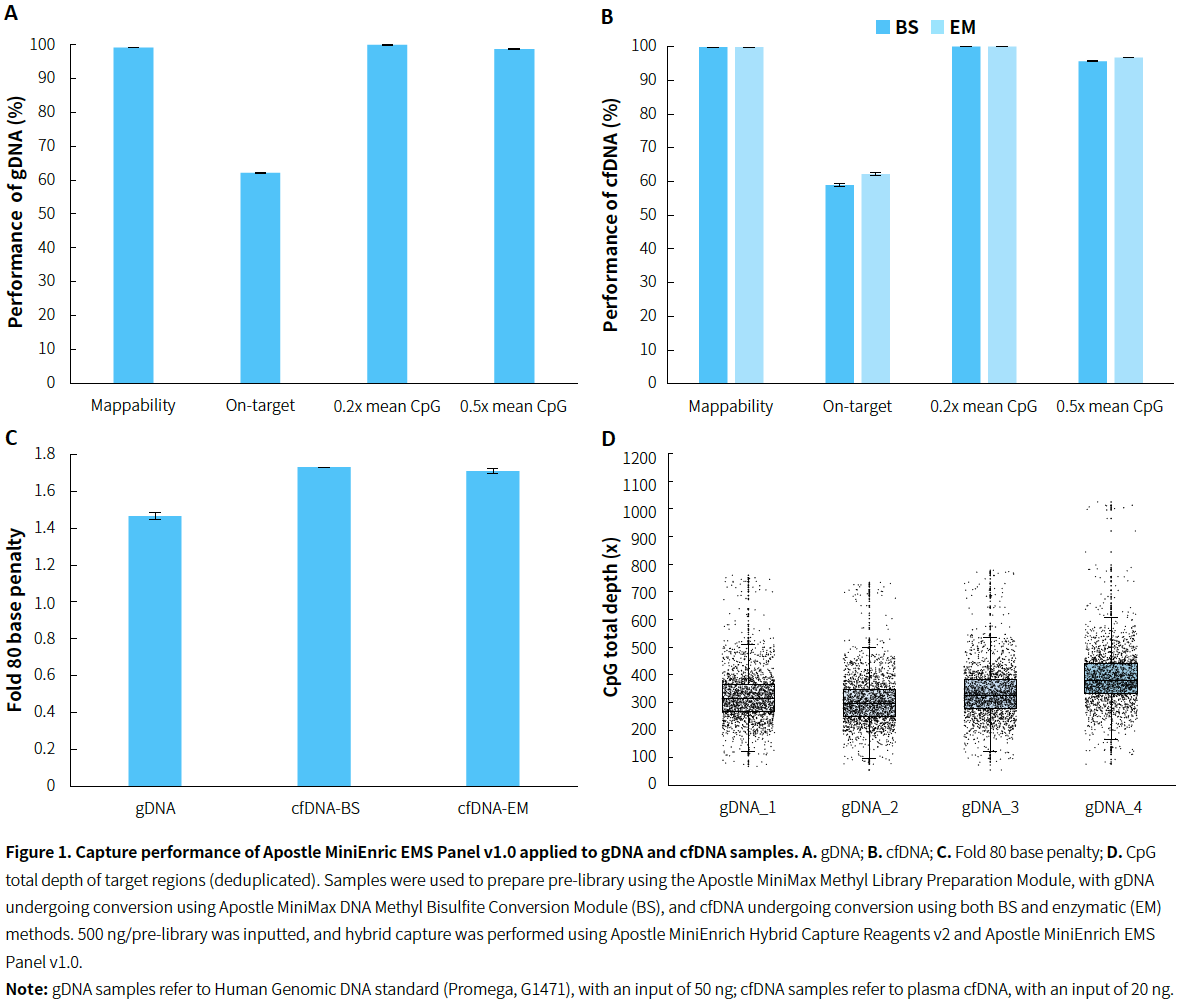
Compatible with Different Sample Types
|
|
Clinical Sample Detection Performance
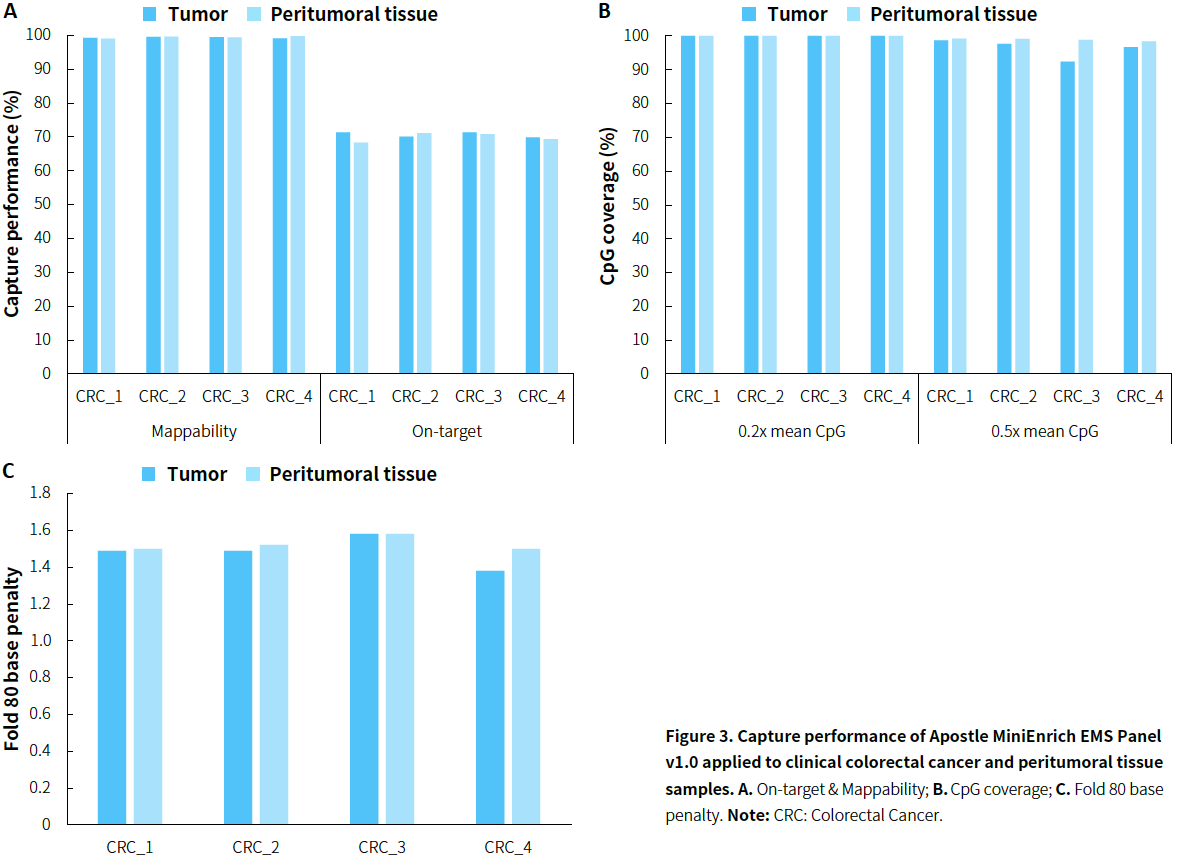
- Colorectal Cancer
|
|
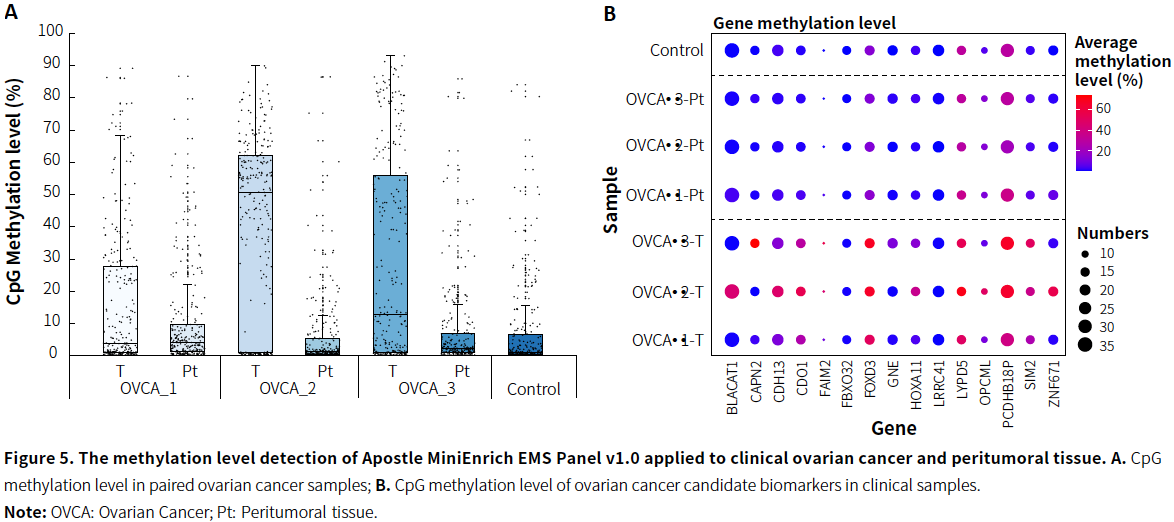
- Ovarian Cancer
|
|
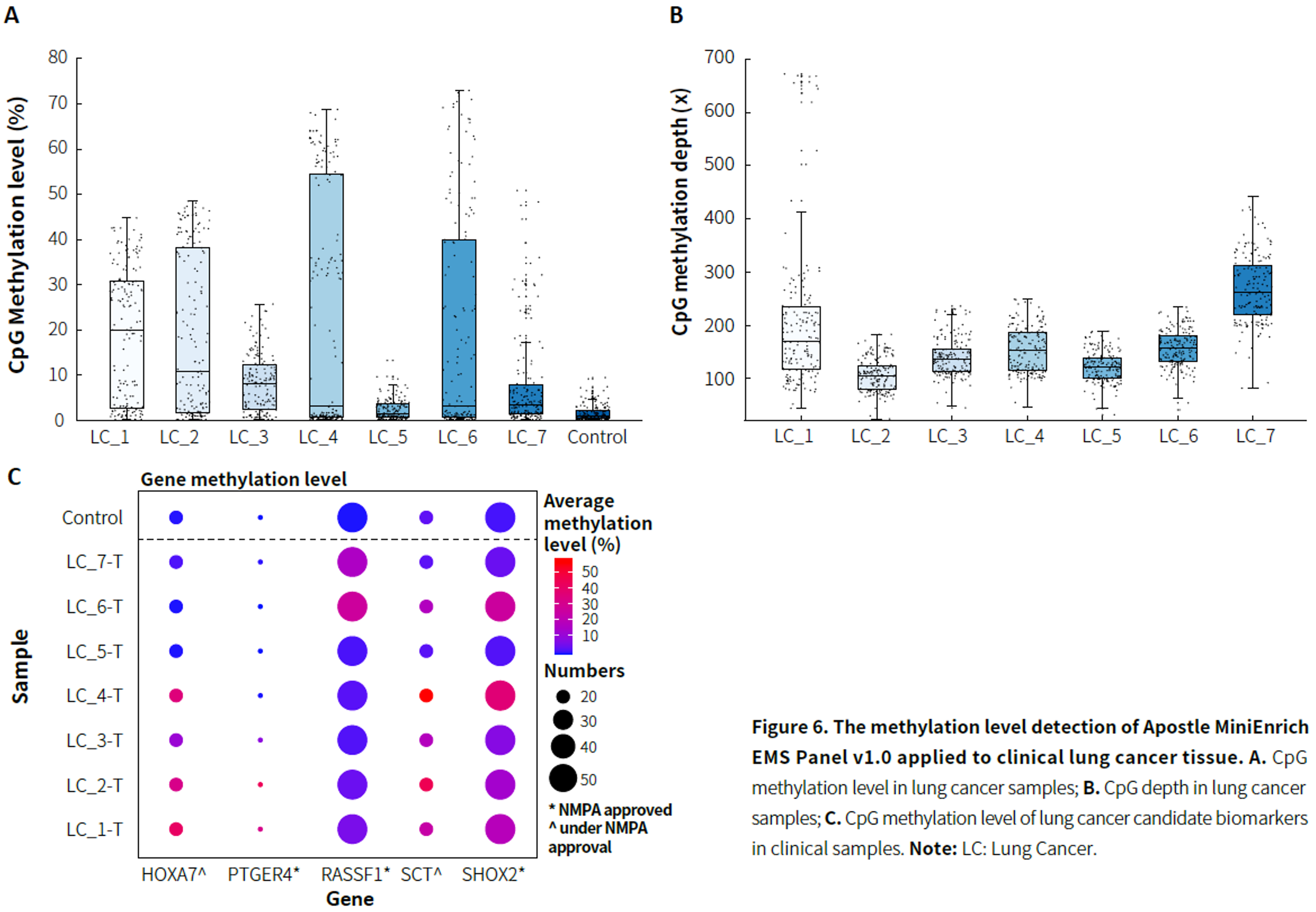
- Lung Cancer
|
|