Apostle technologies have been applied in many world-class R&D studies, clinical laboratory settings, and public health response and surveillance.
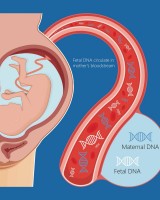
This page lists some of the examples in NIPT (Noninvasive Prenatal Testing).
For a complete list of applications citing Apostle technologies, including publications and customer testimonials, see References.
Noninvasive Prenatal Screening for Common Fetal Aneuploidies Using Single-Molecule Sequencing. Yeqing Qian, Yongfeng Liu, Kai Yan, et al. Laboratory Investigation Volume 103, Issue 4, April 2023, 100043
(Note: Apostle MiniMax technology is used in this study.)
Amplification biases caused by next-generation sequencing (NGS) for noninvasive prenatal screening (NIPS) may be reduced using single-molecule sequencing (SMS), during which PCR is omitted. Therefore, the performance of SMS-based NIPS was evaluated. We used SMS-based NIPS to screen for common fetal aneuploidies in 477 pregnant women. The sensitivity, specificity, positive predictive value, and negative predictive value were estimated. The GC-induced bias was compared between the SMS- and NGS-based NIPS methods. Notably, a sensitivity of 100% was achieved for fetal trisomy 13 (T13), trisomy 18 (T18), and trisomy 21 (T21). The positive predictive value was 46.15% for T13, 96.77% for T18, and 99.07% for T21. The overall specificity was 100% (334/334). Compared with NGS, SMS (without PCR) had less GC bias, a better distinction between T21 or T18 and euploidies, and better diagnostic performance. Overall, our results suggest that SMS improves the performance of NIPS for common fetal aneuploidies by reducing the GC bias introduced during library preparation and sequencing.
(Materials and Methods section) - cfDNA was extracted from 0.6 mL of maternal plasma using Apostle MiniMaxTM High-Efficiency cfDNA Isolation Kit (ref. number: A17622-50; Apostle) according to the manufacturer’s instructions.
Segmental duplication as potential biomarkers for non-invasive prenatal testing of aneuploidies.
Chen et al. EBioMedicine August 11, 2021; https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(21)00328-5/fulltext
We developed a computational program whereby available SD regions can be processed and analyzed efficiently for their potential use as biomarkers of the aneuploidy of interest. For the five common aneuploidies, i.e., trisomy 13, 18, 21, and two sex chromosome aneuploidies, a total of 21,772 candidate SD biomarker sequences together with their corresponding primer/probe sets were generated. The primer/probe sets were tested using a real-time PCR-based multicolour melting curve analysis for simultaneous detection of the five common aneuploidies, and yielded 100% clinical sensitivity and 99.64% specificity when subjected to a clinical evaluation. Following the observations that the SD biomarkers for aneuploidy could be better detected by digital PCR with improved accuracy, we established a noninvasive prenatal testing protocol for trisomy 21 and attained 100% concordance with next generation sequencing.
Our study confirmed that SD regions are preferred biomarkers for aneuploidy detection and in particular SD-based digital PCR could find potential use for NIPT of trisomy. A similar strategy can be applied to other chromosomal abnormality and genetic disorders.
The cfDNA was isolated from 1 mL of each plasma sample by the Apostle MiniMax™ High Efficiency cfDNA Isolation Kit (Apostle Inc, San Jose, CA) in line with the manufacturer's instructions.
The SneakPeek Early Gender DNA Test
SneakPeek by Gateway Genomics was founded with the goal to make DNA-based prenatal and pediatric information accessible and affordable for parents everywhere.
The SneakPeek Early Gender DNA Test is designed to specifically focus on fetal sex and return just one answer – male or female. This enables the test to be run on magnitudes smaller volumes of blood than the typical non-invasive prenatal test. When maternal blood samples arrive at SneakPeek Labs, extracted cell-free fetal DNA is run through real-time quantitative PCR to detect with a sensitivity down to a single Y chromosome. If Y chromosomes are found, the result is a boy. If they are absent, the baby is a girl. The test is run in 3 hours with 99.9% accuracy¹.
The SneakPeek Early Traits DNA Test lets parents find out what their infant or child will look like as an adult, predicted nutrition levels and sleep behavior. The DNA collection process is simple with a rub of the inner cheek using a swab, no blood samples are required for this test making it easy for parents. When the DNA samples arrive at SneakPeek Labs, genotyping method is used to analyze the differences in DNA and determine which traits an individual may have as a result.
(Note: Apostle is a technological product provider to SneakPeek by Gateway Genomics).
¹In a recent large-scale study, SneakPeek accurately determined fetal sex in 99.9% of 1,029 pregnant women between 7-37 weeks gestational age. In a separate clinical study run in 2021, SneakPeek accurately determined fetal sex in 75 out of 75 pregnant women at 7 weeks into pregnancy.
Quantifying Fetal DNA in Maternal Blood Plasma by ddPCR Using DNA Methylation.
E. Hall, T. Riel, M. Ramesh, M. Gencoglu, O. Mikhaylichenko, R. Dannebaum, S. Margeridon, M. Herrera. Bio-Rad Laboratories, Pleasanton, CA. Association for Molecular Pathology 2022 Annual Meeting Abstracts. J Mol Diagn 2022, 24:S1 Abstract G072.
Introduction: The proportion of cell-free DNA (cfDNA) circulating in maternal blood that originates from the fetus, the fetal fraction, is an important quality control metric when performing tests on fetal-derived cfDNA. Epigenetic differences produce dissimilar DNA methylation patterns, allowing for leveraging regions of high methylation contrast using methylation-sensitive restriction enzyme (MSRE) digestion to quantify fetal and maternal DNA via droplet digital PCR (ddPCR). This advancement positions ddPCR as a faster and less expensive alternative to next-generation sequencing (NGS) for fetal fraction estimation.
Methods: Assays were designed to target MSRE- compatible regions with high methylation contrast between maternal and fetal cfDNA. Fetal assays targeted sites hypermethylated in fetal cfDNA and maternal assays targeted sites hypermethylated in maternal cfDNA. The assay multiplex was tested against contrived and clinical samples using an in-droplet MSRE-ddPCR workflow. The reaction mix was dropletized to create about 20,000 droplets per 24-μL reaction, thermocycled, and analyzed in a QX ONE instrument. The thermocycling profile included a 45-minute MSRE incubation step prior to PCR amplification. Contrived samples were constructed by spiking DNA-free plasma with micrococcal nuclease-digested DNA from an amniotic fluid cell line ("fetal" component) and a B-lymphocyte cell line ("maternal" component). Clinical samples were remnant diagnostic samples with existing NGS non-invasive prenatal testing results attached. DNA was extracted from all samples with the Apostle MiniMax kit on the KingFisher Flex.
Results: From an initial set of 15 assays, a final five-assay multiplex was produced following amplicon sequencing with NGS and ddPCR screening. Although amplicon sequencing did not completely predict ddPCR performance and non- specific interactions, it was valuable for guiding the final ddPCR screen. The five-assay multiplex, consisting of three fetal assays and two maternal assays, produced an excellent linear response against contrived samples from 0% to 25% fetal fraction (R2 >0.99). Similarly, a high correlation was observed between ddPCR-estimated fetal fraction and NGS fetal fraction for a set of clinical samples (n=6, plus two non- pregnant controls, R2 >0.94).
Conclusions: As an epigenetic trait, DNA methylation is a useful way to discriminate between otherwise highly similar DNA sequences in an efficient and effective manner. Leveraging DNA methylation may be done with minimal impact to the standard ddPCR workflow. The high sensitivity, speed, and direct quantification of ddPCR make it an attractive alternative to NGS for fetal fraction estimation.
High-resolution DNA size enrichment using a magnetic nano-platform and application in non-invasive prenatal testing.
Zhang et al. Analyst. July 2020, 145, 5733-5739 (PDF)
Precise DNA sizing can boost sequencing efficiency, reduce cost, improve data quality, and even allow sequencing of low-input samples, while current pervasive DNA sizing approaches are incapable of differentiating DNA fragments under 200 bp with high resolution (<20 bp). In non-invasive prenatal testing (NIPT), the size distribution of cell-free fetal DNA in maternal plasma (main peak at 143 bp) is significantly different from that of maternal cell-free DNA (main peak at 166 bp). The current pervasive workflow of NIPT and DNA sizing is unable to take advantage of this 20 bp difference, resulting in sample rejection, test inaccuracy, and restricted clinical utility. Here we report a simple, automatable, high-resolution DNA size enrichment workflow, named MiniEnrich, on a magnetic nano-platform to exploit this 20 bp size difference and to enrich fetal DNA fragments from maternal blood. Two types of magnetic nanoparticles were developed, with one able to filter high-molecular-weight DNA with high resolution and the other able to recover the remaining DNA fragments under the size threshold of interest with >95% yield. Using this method, the average fetal fraction was increased from 13% to 20% after the enrichment, as measured by plasma DNA sequencing. This approach provides a new tool for high-resolution DNA size enrichment under 200 bp, which may improve NIPT accuracy by rescuing rejected non-reportable clinical samples, and enable NIPT earlier in pregnancy. It also has the potential to improve non-invasive screening for fetal monogenic disorders, differentiate tumor-related DNA in liquid biopsy and find more applications in autoimmune disease diagnosis.
Next-Generation Digital Polymerase Chain Reaction: High-Dynamic-Range Single-Molecule DNA Counting via Ultrapartitioning.
Eleen Y. Shum, Janice H. Lai, Sixing Li, Haeun G. Lee, Jesse Soliman, Vedant K. Raol, Cavina K. Lee, Stephen P.A. Fodor, H. Christina Fan Anal. Chem. 2022, Publication Date:December 12, 2022 https://doi.org/10.1021/acs.analchem.2c03649
(Note: Apostle MiniMax technology is used in this study.)
ABSTRACT: Digital PCR (dPCR) was first conceived for single-molecule quantitation. However, current dPCR systems often require DNA templates to share partitions due to limited partitioning capacities. Here, we introduce UltraPCR, a next-generation dPCR system where DNA counting is performed in a single-molecule regimen through a 6-log dynamic range using a swift and parallelized workflow. Each UltraPCR reaction is divided into >30 million partitions without microfluidics to achieve single template occupancy. Combined with a unique emulsion chemistry, partitions are optically clear, enabling the use of a three-dimensional imaging technique to rapidly detect DNA-positive partitions. Single-molecule occupancy also allows for more straightforward multiplex assay development due to the absence of partition-specific competition. As a proof of concept, we developed a 222-plex UltraPCR assay and demonstrated its potential use as a rapid, low-cost screening assay for noninvasive prenatal testing for as low as 4% trisomy fraction samples with high precision, accuracy, and reproducibility.
(Methods Section) Cell-free plasma was isolated using the Apostle MiniMax kit (Beckman Coulter) according to the manufacturer’s protocol with an elution volume of 60 μL.
For a complete list of publications citing Apostle technologies, see Publications.