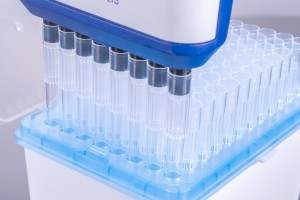
For fast extraction and purification of nucleic acids from various kinds of biological samples, in 96 well format.
- High quality: Equal or better results compared with other brands.
- Lower cost: Guaranteed 30% - 50% less cost than other brands.
- Faster: One button start; Run time is approximately 40 minutes per 96 samples.
- Safer: Integrated with a UV light for unit interior disinfection.
- Cat # A201126-96
Documentation:
Manual: Apostle-MagTouch-2000-manual.pdf
- This link directs to download the user manual for Apostle MagTouch 2000 Nucleic Acids Isolation Automation System.
Protocol: The system has been utilized by our clients to extract cell-free DNA and/or RNAs from various volumes of liquid samples. The system is also easily programmable to adapt with various laboratory settings and needs. Some example protocols are listed below.
Application Note: Application-Note-Apostle-Viral-RNA-Isolation-System.pdf
- This links directs to an Application Note on the Apostle COVID-19 Viral RNA Extraction System, applied in the effective detection of SARS-CoV-2 in over 8 million clinical samples by our clients. Using Apostle system, limit of Detection (LoD) of the downstream detection system of our customer is documented at 3.6 NDU/uL published on the FDA website. The sensitivity and specificity is 99.9% and 99.9% at 3 copies/uL, respectively, published on our customer’s website.
Inclusion in US FDA IVD EUA - Molecular Diagnostic Tests for SARS-CoV-2
Apostle RNA extraction method is included in a US FDA EUA authorized SARS-CoV-2 molecular diagnostic test: Fulgent COVID-19 by RT-PCT Test.
- Weblink for the US FDA EUA summary: https://www.fda.gov/media/138150/download
- Weblink for the US FDA EUA letter: https://www.fda.gov/media/138147/download
More information: Product Data page