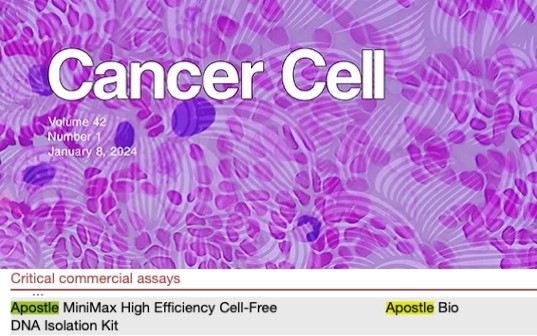
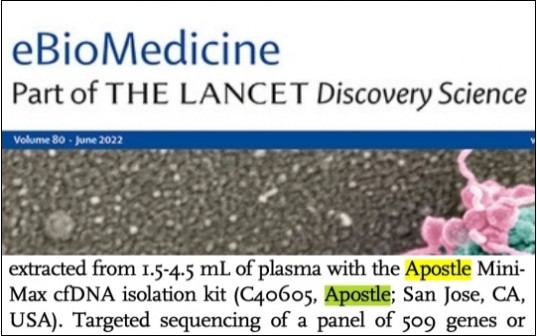
Apostle technologies have been applied in many world-class R&D studies, clinical laboratory settings, and public health response and surveillance. For a complete list of applications citing Apostle technologies, including publications and customer testimonials, see References.
Click the banner below for details of the citing articles.
Apostle technologies have been cited or discussed in the following publications.
Apostle technologies have been cited or used by scientists from the following organizations. Click to read more when there is a publication.
History of Accreditations

Clinical and Public Health Laboratory License, State of California, Department of Public Health 2022-2023, issue date 09/02/2022

CLIA (Clinical Laboratory Improvement Amendments) License, Centers for Medicare & Medicaid Services, the United States Department of Health and Human Services 2021-2023, issue date 08/18/2021

Clinical and Public Health Laboratory License, State of California, Department of Public Health 2021-2022, issue date 09/03/2021